The Centers for Medicare & Medicaid Services (CMS) has revised the focused infection control survey protocol.[1] Now, for the first time, CMS requires surveyors to review the care actually provided to a sample of residents (and among a sample of staff). This important change, supported by the Center for Medicare Advocacy (Center), may result in the citation of more infection control deficiencies than prior focused infection control surveys, which appeared to be limited in scope, short in duration (one surveyor conducted several surveys in one day), and more focused on paperwork compliance than on observing whether staff providing care to residents correctly and consistently followed longstanding infection control practices.
Accurate citing of deficiencies is a key component of the oversight system for nursing facilities. If deficiencies are not cited, facilities will operate under the assumption they have no problems in their infection control practices and they will not make any changes or corrections.
The Center for Medicare Advocacy has contended that the limited number of deficiencies that have been cited during the COVID-19 pandemic is not plausible when so many residents and staff are dying from the coronavirus. This is further unlikely when, as reported by the Government Accountability Office in May 2020, infection control deficiencies before the pandemic were “widespread” and “persistent.”[2]
On August 14, 2020, CMS reported that it had cited more than 180 facilities with immediate jeopardy infection control deficiencies (triple the rate in 2019) and imposed civil money penalties of nearly $10 million, averaging $55,000 for each facility.[3] As we have reported,[4] CMS told residents’ advocates in an August 19, 2020 call that information about these surveys, to date, was reported only on an internal CMS database; these surveys had not been publicly reported. As described below, publicly reported infection control data, released by CMS on August 26, continue to show many fewer immediate jeopardy infection control deficiencies than CMS reports having cited – 48, compared to nearly 180.
However, news reports indicate more deficiencies and penalties than CMS publicly reports. For example, on September 2, 2020, the Baltimore Sun reported that state inspection reports show infection control deficiencies were cited at 64 Maryland nursing facilities, with ten facilities facing fines ranging from $70,000 to $380,000.[5] The Collingswood Rehabilitation and Healthcare Center was fined $275,000 in June. CMS’s cumulative data release of August 26 reports only a March 4 survey at Collingswood that did not cite an infection control deficiency; the release does not show any survey at Collingswood citing an infection control deficiency.
CMS’s August 26 Cumulative Data Release
On August 26, 2020, CMS made public the fourth cumulative release of 25,593 targeted infection control surveys, covering the period March 4 through July 31. The surveys cited 556 infection prevention and control deficiencies in 539 facilities (524 facilities were cited with one deficiency, 13 facilities were cited with two deficiencies, two facilities were cited with three deficiencies).
Of the 556 infection control deficiencies, 48 deficiencies (8.6%) were cited at the immediate jeopardy level.
Although all four releases of infection control surveys and deficiencies show that less than 3% of focused infection control surveys cited deficiencies, an increasing percentage of these deficiencies has been classified as immediate jeopardy.
| Date of CMS Release | Number of Surveys | Number (%) of infection control deficiencies cited | Number (%) of infection control deficiencies classified as immediate jeopardy |
| June 4 | 5,724 | 163 (2.8%) | 1 (1.0%) |
| June 24 | 9,899 | 262 (2.6%) | 4 (1.5%) |
| July 29 | 16,987 | 347 (2.0%) | 22 (6.6%) |
| August 26 | 25,593 | 556 (2.2%) | 48 (8.6%) |
The surveys released in August also show that:
- The scope of deficiencies has shifted. While the first three cumulative releases showed that the largest category of deficiencies was cited as “few,” the fourth cumulative release shows that “some” is now the largest category with respect to scope of deficiencies.
- Most deficiencies (503 of 556 deficiencies, 90.5%) are cited as minimal harm or potential for actual harm (levels D, E, and F), also known as “no harm” deficiencies.
- “No harm” deficiencies (levels D, E, and F) were predominant in all four cumulative releases of infection control surveys.
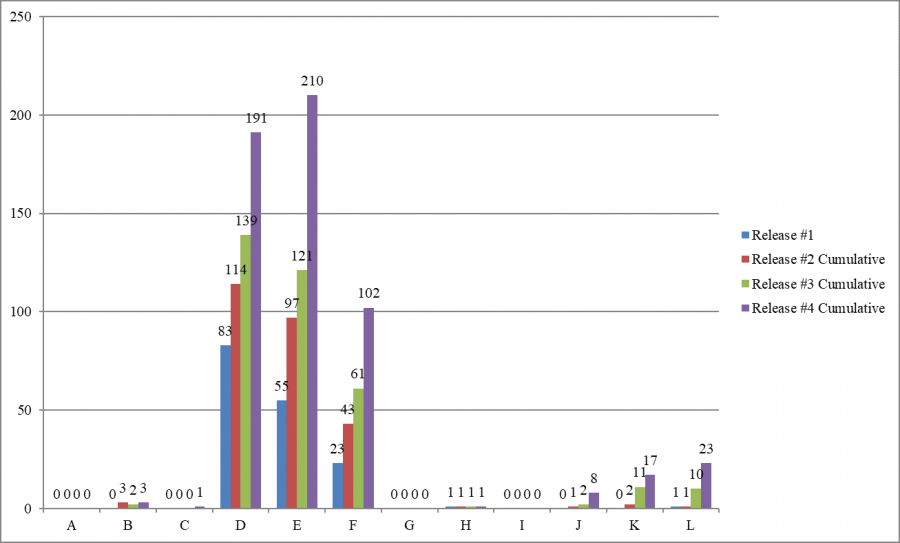
- As in previous releases, Texas cited more infection control deficiencies than any other state – 120 of 556 deficiencies (21.6% of the infection control deficiencies cited nationwide).
Conclusion
The revised survey protocol may lead to a more accurate evaluation of facilities’ failures to comply with infection control requirements.
Although an increasing proportion of the deficiencies is classified as immediate jeopardy, the 48 immediate jeopardy infection control deficiencies publicly reported in August are still far fewer than the nearly 180 number claimed by CMS.
- To read the full report, please go to: https://medicareadvocacy.org/wp-content/uploads/2020/09/Special-Report-Whats-New-In-Infection-Control.pdf
September 3, 2020 – T. Edelman; M. Edelman
[1] CMS, “Interim Final Rule (IFC), CMS-3401-IFC, Additional Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency related to Long-Term Care (LTC) Facility Testing Requirements and Revised COVID-19 Focused Survey Tool,” QSO-20-38-NH (Aug. 26, 2020), https://www.cms.gov/files/document/qso-20-38-nh.pdf.
[2] GAO, “Infection Control Deficiencies Were Widespread and Persistent in Nursing Homes Prior to COVID-19 Pandemic,” GAO-20-576R (May 20, 2020), https://www.gao.gov/assets/710/707069.pdf. The report is discussed in CMA, “GAO on Infection Control Deficiencies in Nursing Facilities Before Covid-19 Pandemic: ‘Widespread,’ ‘Persistent,’ ‘Ignored’” (CMA Alert, May 21, 2020), https://medicareadvocacy.org/gao-widespread-snf-deficiencies/.
[3] CMS, “Trump Administration Has Issued More Than $15 Million in Fines to Nursing Homes During COVID-19 Pandemic” (Press Release, Aug. 14, 2020), https://www.cms.gov/newsroom/press-releases/trump-administration-has-issued-more-15-million-fines-nursing-homes-during-covid-19-pandemic.
[4] CMA, “Responding to CMS Announcement on Nursing Home Enforcement – Infection Control Deficiencies in Nursing Facilities: QCOR Data” (CMA Alert, Aug. 20, 2020), https://medicareadvocacy.org/responding-to-cms-announcement-on-nursing-home-enforcement-infection-control-deficiencies-in-nursing-facilities-qcor-data/.
[5] Meredith Cohn, “Dozens of Maryland nursing homes found with deficient infection control during coronavirus pandemic,” Baltimore Sun (Sep. 2, 2020), https://www.baltimoresun.com/coronavirus/bs-hs-nursing-home-covid-inspections-20200902-ajvxfl76lba6xn6u7bitx7crpm-story.html.
