In March 2020, the Centers for Medicare & Medicaid Services (CMS) suspended the regular survey process and announced that it would conduct limited surveys that focused on infection prevention and control issues.[1] Since June, CMS has made information about these limited surveys publicly available, on the last Wednesday of each month, through the federal website Nursing Home Compare. The Center for Medicare Advocacy has analyzed these survey data each month and issued reports describing the findings. While the total number of surveys has dramatically increased, now totaling more than 40,000, the findings are virtually the same each month.
For six months, CMS has reported that fewer than 3% of the surveys have cited infection control deficiencies, and that the overwhelming majority of deficiencies have been classified as no-harm. A second publicly-available CMS website, Quality, Certification and Oversight Reports (QCOR), reports data about surveys, deficiencies, and civil money penalties. The two publicly-available data sets are not consistent with each other, reporting different information about surveys and deficiencies.[2] Adding to the confusion about surveys and infection control deficiencies is reporting by newspapers and state departments of health about deficiencies and penalties that do not appear on the publicly available federal websites.
Another point of confusion was CMS’s report, on August 14, 2020, that it had cited more than 180 “immediate jeopardy” level deficiencies for infection prevention and control at nursing facilities, triple the rate from 2019, and that it had imposed civil money penalties exceeding $10 million for the deficiencies.[3] CMS acknowledged in a call with nursing home residents’ advocates on August 19 that the data reported on August 14 were available only in an internal CMS database, not on its two publicly-reported databases. Even now, however, nearly three months after CMS’s announcement, the publicly-reported information does not support CMS’s claims about deficiencies or penalties.
Changes in surveys continue. In August, CMS announced that states should gradually begin conducting standard surveys.[4] These survey results are intermingled with the focused infection control surveys.
This report discusses both the sixth cumulative release of focused infection control surveys (reported by CMS on October 28) and, separately, data for the month of September.
Cumulative survey data
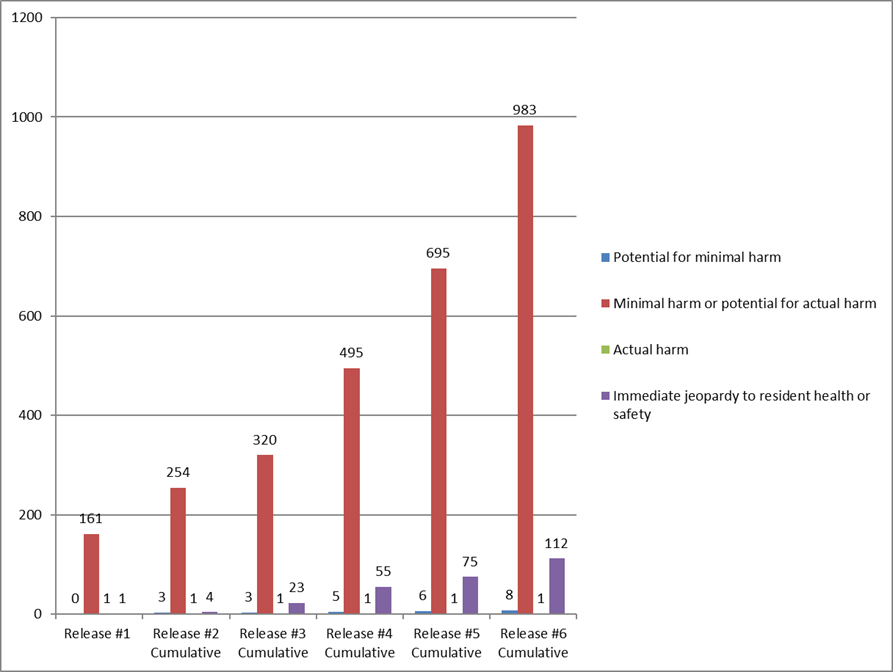
As of October 28, Nursing Home Compare indicates that state survey agencies had conducted 40,144 surveys and cited 1104 deficiencies (2.8%) for infection control (F-880).
The number and percentage of immediate jeopardy infection control survey have increased with each cumulative survey, but remain a small number and percentage of facilities that have had surveys.
| Date of CMS release | Total number of surveys reported | Total number (percent) of infection control deficiencies cited | Total number (percent) of immediate jeopardy deficiencies cited |
| June 4 | 5,724 | 163 (2.8%) | 1 (1.0%) |
| June 24 | 9,899 | 262 (2.6%) | 4 (1.5%) |
| July 29 | 16,987 | 347 (2.0%) | 22 (6.6%) |
| August 26 | 25,593 | 556 (2.2%) | 48 (8.6%) |
| September 30 | 32,681 | 777 (2.4%) | 75 (9.7%) |
| October 28 | 40,144 | 1,104 (2.8%) | 112 (10.1%) |
Most infection control deficiencies are classified as no harm. Of the 1,104 deficiencies, 983 (89.0%) are classified as “no actual harm with the potential for no more than minimal harm” – i.e., “no harm.” Only 112 of the 1104 F-880 deficiencies (10.1%) are classified as immediate jeopardy.
Each of the six cumulative surveys has cited most of the infection control deficiencies as “no harm” – levels D, E, and F.
Scope and Severity of F-880 Deficiencies
Cumulative Data (Oct. 28, 2020)
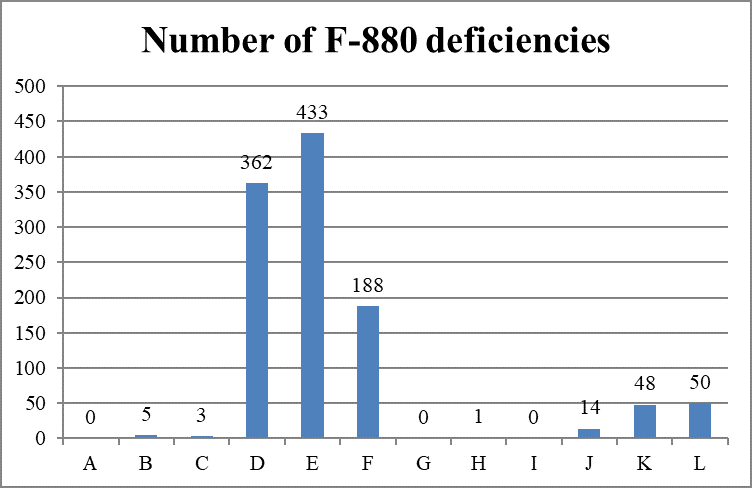
As categorized by the combined scope and severity classifications, most deficiencies (983 of 1,104 deficiencies, 89.0%) are cited as “minimal harm or potential for actual harm” (levels D, E, and F), also known as “no harm.”
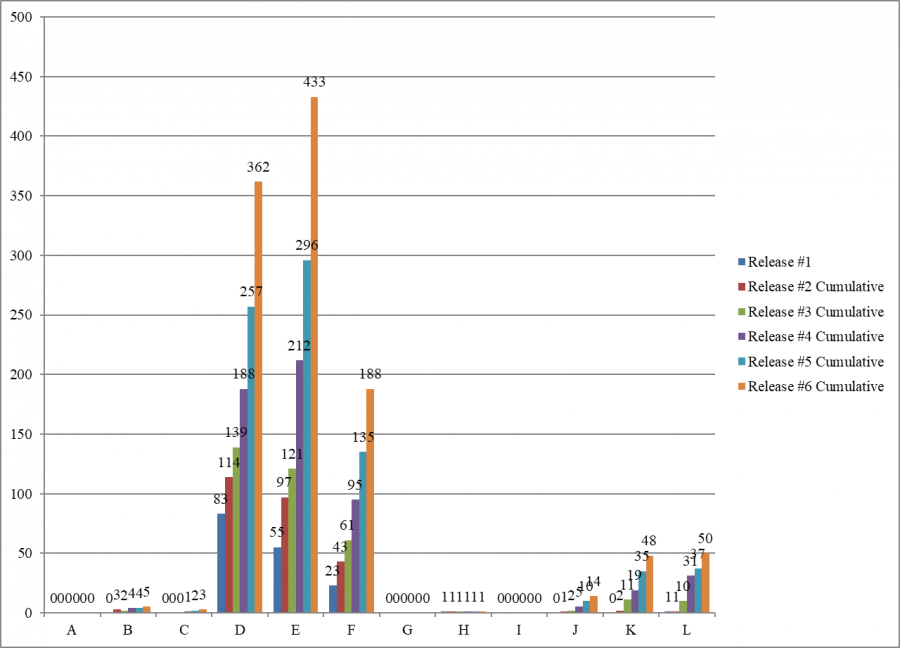
September Surveys
In September, state survey agencies conducted 3855 surveys. Only 27 surveys (.007%) cited an infection control deficiency. All 27 infection control deficiencies were classified as “no harm.”
Conclusion
As COVID-19 cases increase in nursing facilities nationwide and residents and staff die, CMS continues to report that surveys cite few deficiencies and classify most of them as “no-harm,” meaning no enforcement.
November 12, 2020 – M. Edelman, T. Edelman
[1] CMS, “Suspension of Survey Activities,” QSO-20-12-All (Mar. 4, 2020), https://www.cms.gov/files/document/qso-20-12-all.pdf; CMS, ‘Prioritization of Survey Activities,” QSO-20-20-All (Mar. 23, 2020), https://www.cms.gov/files/document/qso-20-20-all.pdf.
[2] “Infection Control Surveys At Nursing Facilities” (CMA Alert, Oct. 29, 2020), https://medicareadvocacy.org/infection-control-surveys-at-nursing-facilities/.
[3] CMS, “Trump Administration Has Issued More Than $15 Million in Fines to Nursing Homes During COVID-19 Pandemic” (Press Release, Aug. 14, 2020), https://www.cms.gov/newsroom/press-releases/trump-administration-has-issued-more-15-million-fines-nursing-homes-during-covid-19-pandemic.
[4] CMS, “Enforcement Cases Held during the Prioritization Period and Revised Survey Prioritization,” QSO-20-35-ALL (Aug. 17, 2020), https://www.cms.gov/files/document/qso-20-35-all.pdf.
