- CMS Releases Proposed 2023 Rule for Medicare Advantage and Part D Plans
- Jimmo Update: CMS Reminds Providers and Contractors of Medicare Coverage to Maintain or Slow Decline
- Skyrocketing COVID-19 Cases Lead to Hospital Struggles Across the Nation
- CMS Again Revises Visitation Guidance in Nursing Facilities
- CMS to Post Nursing Home Staff Turnover and Weekend Staffing Level Information on Care Compare
- FREE WEBINAR | Medicare & Health Care Updates
CMS Releases Proposed 2023 Rule for Medicare Advantage and Part D Plans
On January 6, 2022, CMS released CY 2023 Medicare Advantage and Part D Proposed Rule (CMS-4192-P) and an accompanying Press Release describing the overall rule, and a separate Press Release focusing on Part D prescription drug costs. The proposed rule was published in the Federal Register on January 12, 2022, available here (87 Fed Reg 1842, CMS-4192-P). Comments are due March 7, 2022.
On the whole, this proposed rule signals a renewed dedication to providing needed oversight of Medicare Advantage (MA) and Part D plans – a welcome development. As discussed below, there are a number of provisions that will considerably help consumers, but, in other ways, the proposed rule falls short of providing needed consumer protections. This CMA Alert provides brief summaries of some of the provisions in the proposed rule. The Center will be submitting more detailed comments to CMS, and encourages others to do so as well. We will post our comments on our website prior to the deadline.
Provisions of Proposed Rule
The following are short summaries of some, but not all, of the provisions of the proposed rule, with our added comments where relevant:
- Marketing and Communications – CMS proposes to strengthen oversight of marketing and communications re: MA and Part D plans based, in part, on an increase in beneficiary complaints concerned about (and an increase in TV and print ads related to) the marketing practices of third-party marketing organizations (TPMOs) who sell multiple MA and Part D products; in response, CMS proposes to:
- Make plan issuers responsible for the activities of TPMOs in the same way that they are now responsible for the activities of agents, brokers and other “first tier, downstream or related entities.”
- Require a TPMO to use the following standard disclaimer “prominently displayed on the TPMO’s website and marketing materials, including all print materials and television advertising that meet the definition of marketing [as well as] provided verbally, electronically, or in writing, depending on how the TPMO is interacting with the beneficiary.” (p. 1901):
- “We do not offer every plan available in your area. Any information we provide is limited to those plans we do offer in your area. Please contact Medicare.gov or 1-800-MEDICARE to get information on all of your options.”
- CMS also proposes, among other things, to reinstate inclusion of multi-language inserts in top 15 languages used in the U.S. in specified materials re: availability of translation services (when beneficiaries are provided CMS-required materials, such as the Evidence of Coverage, enrollment form, Annual Notice of Change, and Summary of Benefits)
- Comment: we applaud CMS for this long-overdue enhancement of oversight of TPMOs; further, we appreciate that CMS reinstated the requirements concerning multi-language inserts. We are disappointed, however, that CMS did not roll back other recent revisions to marketing guidelines that, among other things, weaken the distinction between education and marketing events (see, e.g., this CMA Alert discussing the January 2021 final rule)
- MA Network adequacy requirements for new or expanding plans – CMS proposes to require plan sponsor applicants to demonstrate (rather than just attest) that they meet network adequacy requirements for a pending service area at application “and to adopt a time-limited 10-percentage point credit toward meeting the applicable network adequacy standards for the application evaluation.”
- Comment: we support this proposal and agree that it will strengthen CMS’ “oversight of an organization’s ability to provide an adequate network of providers to deliver care to MA enrollees.” (p. 1844). CMS does not, however, reverse recent policy changes that have generally weakened MA network adequacy requirements (for further discussion, see this CMA Alert discussing a June 2020 final rule)
- Past Performance Methodology to Better Hold Plans Accountable for Violating CMS Rules – CMS proposes to include additional criteria in their methodology to determine if it should permit an organization to enter into or expand an existing contract; specifically, CMS proposes to include Star Ratings, bankruptcy issues, and compliance actions
- Comment: We strongly support this enhanced oversight of plan sponsors, but note that MA plan Star Ratings are, by some accounts, unreliable (see, e.g., discussion in an October 2021 CMA Special Report)
- Greater Transparency re: MA Medical Loss Ratio – CMS proposes to reinstate previous requirements (and add some new ones) concerning greater transparency and reporting rules surrounding the medical loss ratio (MLR, or the “percentage, generally representing the percentage of revenue used for patient care rather than for such other items as administrative expenses or profit.” (p. 1902))
- Comment: we support this effort to enhance oversight and reporting of plans’ profit and spending
- Special MA Requirements During a Disaster or Emergency – CMS proposes to clarify the period of time during which MA organizations must comply with special requirements to ensure access for enrollees to covered services
- Calculation of Star Ratings for Certain Measures for 2023 Given Impacts of Pandemic – [star ratings flawed]
- Dual-Eligible Special Needs Plans (D-SNPs):CMS proposes several changes to dual eligible special needs plans (D-SNPs), with the stated aim of improving integration of Medicare and Medicaid programs for those enrolled in the plans. The proposed changes include, among others, the following:
- a requirement that any MA organization offering a D-SNP must establish one or more enrollee advisory committees, which includes enrollees in the plans, in each state in order to solicit direct input on enrollee experiences, such as coordination of services, and health equity for underserved populations
- changes to definitions for fully integrated dual eligible special needs plan (FIDE SNP) and highly integrated dual eligible special needs plan (HIDE SNP)
- Establishing D-SNP-specific contracts
- a requirement that all SNPs include one or more standardized questions on the topics of housing stability, food security, and access to transportation as part of their Health Risk Assessments
- Attainment of Maximum Out-of-Pocket (MOOP) limit – CMS proposes to count accrual of all cost-sharing, regardless of whether it is paid by the beneficiary, Medicaid, other secondary insurance or is unpaid towards the MOOP; the current policy “results in increased State payments of Medicare cost-sharing and disadvantages providers serving dually eligible individuals in MA plans” (p. 1843-4)
- Comment: we support this provision, including for the reasons articulated by CMS that include the negative impact of current policy on access to Medicare providers for dually eligible enrollees
- Part D Price Concessions at the Point of Sale – According to a CMS press release, the agency is “proposing a policy that would require Part D plans to apply all price concessions they receive from network pharmacies to the point of sale, so that the beneficiary can also share in the savings. Specifically, CMS is proposing to redefine the negotiated price as the baseline, or lowest possible, payment to a pharmacy, effective January 1, 2023. This policy would reduce beneficiary out-of-pocket costs and improve price transparency and market competition in the Part D program”.
What’s Missing
For many years, the Center for Medicare Advocacy has pushed for legislative and administrative efforts to address the growing inequities between Medicare Advantage (MA) and traditional Medicare that favor MA, and thus the growing privatization of the Medicare program. These inequities include payment/spending, coverage of services, enrollment opportunities, and education/promotion of MA over other coverage options.
As discussed in our September 2021 CMA Special Report concerning the 2022 Medicare & You handbook, we appreciate the steps that the current administration has taken to reverse the bias in favor of MA plans in recent additions, and we outlined where further work is necessary. As noted above, we also recognize that the proposed 2023 Part C and D rule is a welcome departure from recent de-regulatory efforts and hands-off oversight of the MA program, and strongly support many of the provisions proposed.
Through this propose rule, however, CMS does not make every effort within their authority to address the imbalance between MA and traditional Medicare, nor does it impose the greater level of oversight of private Medicare plans that is required to ensure both adequate consumer protections and safeguarding of program spending.
In December 2020, the Center issued a Transition Memorandum for the incoming administration outlining a range of proposed administrative actions that would improve access to coverage and quality of care for all people who rely on Medicare. This included a number of MA and Part D related proposals. Many of these suggestions, which were developed in response to our and others’ experience assisting Medicare beneficiaries, remain unaddressed in this proposed rule. In addition to issues of payment equity (which may be addressed in other pending rules), we urge CMS to take further action in these areas (addressed in the Transition Memo), including the following:
- Oversight of MA Coverage and Care Denials, including restricting rampant use of prior authorization (PA) (the proposed rule only contains a request for information concerning PA for hospital transfers to post-acute care settings during a public health emergency – we assert that the problems created by PA are much more widespread and must be addressed more broadly)
- Revising MA flexibilities re: uniformity standards, meaningful differences that make it more difficult for beneficiaries to make informed decisions
- MA network adequacy – as noted above, the proposal re: tighter restrictions for new entrants is welcome, but the overall thresholds were weakened in recent years
- No new rights for beneficiaries re: Special Enrollment Periods (e.g. for mid-year provider terminations by plans)
- No rescission of allowance of MA plans to apply step-therapy to Part B drugs
- No reinstating of reporting requirements re: appeals
- No expansion of services that limit cost-sharing to traditional Medicare levels
- Marketing Communications Guidelines – while the proposed changes noted above are both welcome and necessary, CMS should reverse the significant changes to the guidelines that have weakened protections in recent years, and add new requirements; for example, as noted above, CMS should:
- Rescind rules weakening marketing v. educational events
- Impose requirements re: MA descriptions of supplemental benefits for the chronically-ill (SSBCI)
Conclusion
As a whole, this proposed rule is an important step in the right direction with respect to imposing greater oversight of Medicare Advantage plans. More is needed, however, in order to adequately protect plan enrollees and the Medicare program.
Jimmo Update: CMS Reminds Providers and Contractors of Medicare Coverage to Maintain or Slow Decline
Skilled nursing care and skilled therapy services for beneficiaries who need skilled care to maintain function or to prevent or slow decline is covered by Medicare. That is the message of reminders issued by the Centers for Medicare & Medicaid Services (CMS) to Medicare providers as well as to Medicare Administrative Contractors.[1] CMS disseminated the information in its December 2, 2021 MLN Connects Newsletter.[2] Furthermore, CMS directed providers and contractors to visit the Jimmo Settlement Agreement webpage for further details.
The CMS statement follows:

The Center for Medicare Advocacy and Vermont Legal Aid brought the Jimmo v. Sebelius class action lawsuit on behalf of beneficiaries who were being denied Medicare coverage for skilled care on the basis that they were not improving or did not demonstrate potential for improvement. In 2013, the federal district court approved a settlement agreement that confirmed Medicare coverage should be determined by a beneficiary’s need for skilled care, not the individual’s potential for improvement; Medicare covers skilled care to maintain an individual’s condition or slow decline.[3] Essentially, improvement or progress is not necessary as long as skilled care is required. The Jimmo standards apply to home health care, nursing home care, outpatient therapies, and, to a certain extent, for care in Inpatient Rehabilitation Facilities/Hospitals.[4]
Check out a collection of Jimmo resources from the Center for Medicare Advocacy here .
___________________
[1] CMS. MLN Connects® Newsletter (2021-12-02-MLNC). CMS. (December 2, 2021). Available at: https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2021-12-02-mlnc#_Toc89267937
[2] CMS. MLN Connects® Newsletter. CMS. (n.d.). Available at: https://www.cms.gov/Outreach-and-Education/Outreach/FFSProvPartProg/Provider-Partnership-Email-Archive
[3] CMA. Jimmo v. Sebelius Factsheet: Medicare Skilled Nursing Facility Coverage Does Not Require Improvement. Center for Medicare Advocacy. (May 20, 2021). Available at: https://medicareadvocacy.org/jimmo-v-sebelius-factsheet-medicare-skilled-nursing-facility-coverage-does-not-require-improvement/
[4] CMA. Improvement Standard and Jimmo News. Center for Medicare Advocacy. (May 15, 2020). Available at: https://medicareadvocacy.org/medicare-info/improvement-standard/
Skyrocketing COVID-19 Cases Lead to Hospital Struggles Across the Nation
The United States is facing the biggest surge of COVID-19 cases to date. According to the Centers for Disease Control and Prevention (CDC), on Tuesday, January 11, there were 797,216 new cases of the disease.[1] To put this into context, during the summer of 2020, the peak number of new COVID cases per day was 77,000 – a more than tenfold increase since that time.[2] Cases are also geographically widespread, with 99.13 percent of the counties in the U.S. experiencing “high” levels of community transmission of COVID-19.[3] The CDC measures transmission level by examining the total new cases of COVID-19 infections reported over the past 7 days and the percent of positive COVID tests for the county over the past 7 days.[4] The higher number of new cases and higher percent positivity correspond to higher levels of community transmission.
In Connecticut, for example, every county is rated with a high transmission level. While 79.4 percent of the state’s population of five-year-olds and over are vaccinated, Connecticut’s Department of Public Health is reporting a 24 percent positivity rate.[5] Furthermore, 82 percent of hospitals beds in the state are filled, and over a quarter of them are being used for COVID-19 patients. Connecticut’s utilization statistics are slightly higher than the national average of 79.15 percent of beds being filled, and just over one-fifth being used for those with COVID-19.[6]
The Center for Medicare Advocacy analyzed state-aggregated data for hospital utilization provided by the Department of Health & Human Services (HHS).[7] We highlight the top 25 states with the highest percent of inpatient population with suspected or confirmed COVID-19. Maryland ranked highest, with 42 percent of hospital patients having or suspected to have COVID and inpatient utilization at 87 percent. Connecticut ranks 7th, with 32 percent of hospital patients having or suspected to have COVID-19.
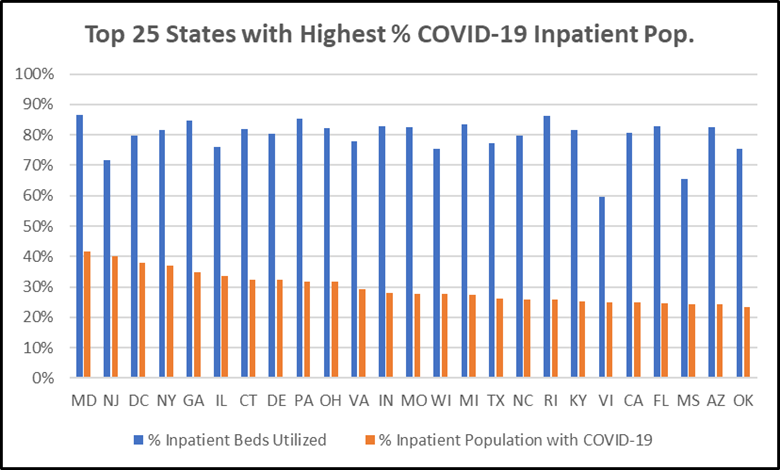
Exacerbating this strain is the fact that hospitals are facing critical staff shortages. All but five states, along with Puerto Rico, the Virgin Islands, and American Samoa, reported hospitals with critical staff shortages.[8]
___________________
[1] CDC. Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, by State/Territory. Centers for Disease Control and Prevention. (Updated January 12, 2022). Available at: https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
[2] CDC. Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, by State/Territory. Centers for Disease Control and Prevention. (Updated January 12, 2022). Available at: https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
[3] CDC. Covid-19 County Check Tool. Centers for Disease Control and Prevention. (September 30, 2021). Available at: https://www.cdc.gov/coronavirus/2019-ncov/more/aboutcovidcountycheck/index.html
[4] CDC. Covid-19 County Check Tool. Centers for Disease Control and Prevention. (September 30, 2021). Available at: https://www.cdc.gov/coronavirus/2019-ncov/more/aboutcovidcountycheck/index.html
[5] CT.gov. Connecticut COVID-19 data tracker. CT.gov. (Updated January 11, 2022). Available at: https://portal.ct.gov/Coronavirus/COVID-19-Data-Tracker
[6] HHS. Hospital Utilization. HHS Protect Public Data Hub. (Updated January 12, 2022). Available at: https://protect-public.hhs.gov/pages/hospital-utilization
[7] U.S. Department of Health & Human Services. Covid-19 reported patient impact and hospital capacity by State. HealthData.gov. (Updated January 12, 2022). Available at: https://healthdata.gov/dataset/COVID-19-Reported-Patient-Impact-and-Hospital-Capa/6xf2-c3ie
[8] HHS. Hospital Utilization. HHS Protect Public Data Hub. (Updated January 12, 2022). Available at: https://protect-public.hhs.gov/pages/hospital-utilization
CMS Again Revises Visitation Guidance in Nursing Facilities
In March 2020, at the beginning of the coronavirus pandemic, the Centers for Medicare & Medicaid Services (CMS) barred visitors from nursing facilities. Since then, it has issued multiple revisions to its guidance. On November 12, 2021, CMS wrote, “Visitation is now allowed for all residents at all times.”[1] CMS prohibited facilities from limiting “the frequency and length of visits for residents, [and] the number of visitors” or from requiring “advance scheduling of visits.” Frequently Asked Questions (FAQs) issued on December 23, 2021 contradicted the November guidance and allowed nursing facilities, demonstrating “good faith efforts . . . to facilitate visitation,” to restructure their visitation policies, to ask visitors to stagger their visits, and to limit the number of visitors.[2] On January 6, 2022, CMS revised the December FAQs again,[3] as discussed below. The changing guidance on visitation reinforces the need for enactment of the Essential Caregivers Act of 2021, H.R. 3733,[4] to recognize a category of individuals – “essential caregivers” – who may be present in a facility, providing assistance and support, during any public health emergency, despite limitations otherwise imposed on visitors.
In the January 6 revisions to the FAQs, CMS reiterates that visitation can occur during the pandemic, but also stresses that “States may instruct nursing homes to take additional measures to make visitation safer.” States have begun to impose requirements for visitors. For example, on January 10, Rhode Island announced new regulations[5] requiring visitors “to either be vaccinated or provide proof of a negative COVID-19 test.”[6]
CMS also now encourages facilities “to consult with state and local health departments when outbreaks occur to determine when modifications to visitation policy would be appropriate.” Facilities should document these discussions and “actions they took to attempt to control the transmission of COVID-19.”
The January revisions to the FAQs add three entirely new questions and answers, at the end of the FAQs. (Note: all revisions are in red italics.) Question 10 confirms that facilities should “continue to permit visitation,” despite the spikes in infections caused by the Omicron variant:
While CMS is concerned about the rise of COVID-19 cases due to the Omicron variant, we’re also concerned about the effects of isolation and separation of residents from their loved ones. Earlier in the pandemic we issued guidance for certain limits to visitation, but we’ve learned a few key things since then. Isolation and limited visitation can be traumatic for residents, resulting in physical and psychosocial decline. So, we know it can lead to worse outcomes for people in nursing homes. Furthermore, we know visitation can occur in a manner that doesn’t place other residents at increased risk for COVID-19 by adhering to the practices for infection prevention, such as physical distancing, masking, and frequent hand hygiene. There are also a variety of ways that visitation can be structured to reduce the risk of COVID-19 spreading. So, CMS believes it is critical for residents to receive visits from their friends, family, and loved ones in a manner that does not impose on the rights of another resident. Lastly, as indicated above, facilities should consult with their state or local public health officials, and questions about
visitation should be addressed on a case by case basis.
Question 11 reiterates federal regulations, 42 C.F.R. §483.10(f)(2) and (4), as “explicitly” stating that residents “have the right to make choices about significant aspects of their life in the facility and the right to receive visitors as long as it doesn’t infringe on the rights of other residents.”
Question 12 confirms that “visitation can occur regardless of the visitor’s vaccination status.” It also suggests “extra precautions” that facilities “can and should take,” such as outdoor visits; “creating dedicated visitation space indoors;” permitting visitors in resident rooms when the roommate is not present; encouraging the use of a well-fitting mask, “preferably those with better protection, such as surgical masks or KN95;” offering surgical masks or KN95 masks; “increasing air-flow and ventilation,” and more.
___________________
[1] CMS, “Nursing Home Visitation – COVID-19 (REVISED),” QSO-20-39-NH (revised 11/12/2021), https://www.cms.gov/files/docuent/qso-20-39-nh-revised.pdf, discussed in CMA, “CMS Revises Visitation Rules for Nursing Facilities” (CMA Alert, Nov. 18, 2021), https://medicareadvocacy.org/cms-revises-visitation-rules-for-nursing-facilities/. See also CMA, “Updated Factsheet/CMS Nursing Home Visitation Guidance” (Dec. 2, 2021), https://medicareadvocacy.org/new-factsheet-cms-nursing-home-visitation-guidance/
[2] CMS, “Nursing Home Visitation Frequently Asked Questions (FAQs)” (Dec. 23, 2021), https://www.cms.gov/files/document/nursing-home-visitation-faq-1223.pdf, discussed in CMA, “CMS Revises November Visitation Guidance after Nursing Home Industry Calls on CMS to Allow Facilities to ‘Limit, Restrict, or Prohibit Visitors’” (CMA Alert, Dec. 23, 2021), https://medicareadvocacy.org/cms-revises-november-visitation-guidance-after-nursing-home-industry-calls-on-cms-to-allow-facilities-to-limit-restrict-or-prohibit-visitors/
[3] CMS, “Nursing Home Visitation, Frequently Asked Questions (FAQs)” (Jan. 6, 2022), https://www.cms.gov/files/document/nursing-home-visitation-faq-1223.pdf
[4] https://www.congress.gov/bill/117th-congress/house-bill/3733?s=1&r=81
[5] 216-RICR-40-10-27.5, link to regulation provided in News Release, note 6, infra
[6] Rhode Island Governor Dan McKee, “Governor McKee Announces New Visitation Measures to Protect Vulnerable Residents in Nursing Homes, Assisted Living Facilities” (News Release, Jan. 10, 2022), https://governor.ri.gov/press-releases/governor-mckee-announces-new-visitation-measures-protect-vulnerable-residents
CMS to Post Nursing Home Staff Turnover and Weekend Staffing Level Information on Care Compare
This month, the Centers for Medicare & Medicaid Services (CMS) will begin posting nursing staff turnover information and weekend staffing levels on the federal website Care Compare.[1] Specifically, CMS will post:
Staff Turnover: “The percent of nursing staff and number of administrators that stopped working at the nursing home over a 12-month period.” CMS will post:
- “The percent of [registered nurse] RN staff that left the facility over the last year.
- “The percent of total nurse staff that have left the facility over the last year.
- “The number of administrators that have left the facility over the last year.”
Weekend Staffing: “The level of total nurse and registered nurse RN staffing on weekends provided by each nursing home over a quarter.”
CMS will post the level of weekend RN and total nurse staff (RN, licensed practical nurse (LPN), certified nurse aide (CNA), “reported in terms of the average number of RN and total nurse hours worked per resident per day on weekends.”
Beginning in July 2022, CMS will use the staff turnover and weekend staffing information in its Nursing Home Five Star Quality Rating System.
CMS cites reports by the HHS Inspector General about staffing and Care Compare. One report found that lower staffing levels on weekends are not reported on the federal website;[2] the other called for posting of information on staff turnover and tenure,[3] as explicitly required by the Affordable Care Act.[4]
In its memorandum, CMS identifies a number of factors that may suggest why lower turnover rates are associated with higher quality of care for residents. For example, staff may be more familiar with residents and more able to identify changes in residents more quickly; staff may be more familiar with facility policies and procedures; and lower administrator turnover may reflect “greater leadership stability, direction, and operations, which may help staff provide care more consistently or effectively to residents.”
___________________
[1] CMS, “Nursing Home Staff Turnover and Weekend Staffing Levels,” QSO-22-08-NH (Jan. 7, 2022), https://www.cms.gov/files/document/qso-22-08-nh.pdf
[2] HHS Office of Inspector General, “Some Nursing Homes’ Reported Staffing Levels in 2018 Raise Concerns; Consumer Transparency Could Be Increased” (Data Brief), OEI-04-18-00450 (Aug. 20, 2020), https://oig.hhs.gov/oei/reports/OEI-04-18-00450.pdf
[3] HHS Office of Inspector General, “CMS Use of Data on Nursing Home Staffing: Progress and Opportunities To Do More,” OEI-04-18-00451 (Mar. 2021), https://oig.hhs.gov/oei/reports/OEI-04-18-00451.pdf
[4] 42 U.S.C. §§1395i-3(i)(1)(A)(i), 1396r(i)(1)(A)(i), Medicare and Medicaid, respectively
FREE WEBINAR | Medicare & Health Care Updates
Wednesday, January 27, 2022 @ 1 – 2:30 PM EST
This webinar will provide an overview of Medicare issues in 2022, including potential legislative and administrative changes. We will highlight key issues to watch in the coming year, including pandemic-related policy changes. We will feature a discussion about the need for Medicare coverage of audiology care with Dr. Frank Lin, Director of the Cochlear Center for Hearing and Public Health, Johns Hopkins University.
Presented by Center for Medicare Advocacy Associate Director David Lipschutz, Policy Attorney Kata Kertesz, and Special Guest Dr. Frank Lin, John Hopkins University, as well as SMP Outreach Specialist & Case Manager Sandy Morales with an update on fraud trends.
Register Now: https://attendee.gotowebinar.com/register/7676601484705606158
